Cells and Batteries-Amazing things you did not know about
Table of Contents
ToggleDifference between Cells and batteries
Electric cell and battery first invented by alessandro volta. Cell and battery which we use as source of emf. Cells and battery both are work on the principle of energy conversion.
Both are convert chemical energy into an electrical energy. According to the principle, when two different type of conducting materials are immersed in an electrolyte. As a result of a chemical reaction, the charge in this solution gets separated.
This charges is accumulated by the conducting material.such charged material is called electrodes.The electrode which is charged with positive charge is called anode and the electrode which is charged with negative charge is called cathode.
In this way, due to accumulation of two opposite charges, a potential difference is established between the two electrodes.due to this potential difference,the chemical energy of the cell and battery both is converted into electrical energy.
cell is a single unit but battery is the combination of various cells.so a single unit cell does not provide sufficient level of voltage or current but battery obtained desired voltage or current for load.
Some Internal Fact of Cells and Batteries.
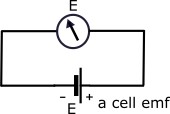
Indivisual Cell Emf:-
an open circuit condition the voltage of a cell is called emf of a cells.this is represented by E.it is measured by high resistance voltmeter.
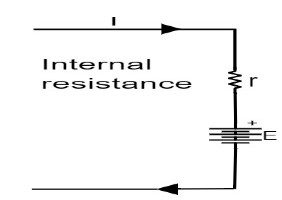
Cell Internal Resistance
just as a circuit has a resistance to an external load,a cell also has an internall resistance that opposes the flow of current.it is denoted as r and measured in ohms.this resistance acts in a series of cells
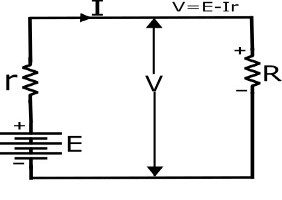
Cell Terminal Voltage
when a load is connected in the external circuit of the cell,the current flows I in the circuit.due to this there is voltage drop Ir across in the internal resistance of the cell.due to this reason,the cell emf E drops and the terminal voltage V is alaways less then the cell emf E.
can show it like this
V=E-Ir—— (I)
and due to external resistance
V=IR—- (II)
from (I) and (II) we get
E-Ir=IR
E=IR+Ir
=I(R+r)
the cell consists of an assembly of electrodes,separators,electrolyte,container and terminal.practically the voltage of a single cell is not sufficient therefore various cells are connected in series.parallel or grouping to obtaine the required voltage lavel.when two or more cells are connected is called battery.
Some Components of Cell and Battery
Electrode
an electrodes is a metallic compound or metal.there are two types of electrodes in a cell or metal.there are two types of electrodes in a cell or battery,positive electrode and negative electrode.positive electrode called anode and negative electrode called cathode.
any anode,it must have some special properties:-
- its efficiency as reducing agent
- it should be high coulombic output(Ah/g)
- anode material have good conductivity.
- its stability should be better.
- ease of fabrication
- cost should be low
the choice of chetode should be such it must:-
- it must be an efficient oxidizing agent.
- be stable when in contact with the electrolyte
- it must be a usefull working voltage.
Electrolyte
electrolyte provides the medium for transfer of charge in between positive and negative electrodes.the electrolyte of a cell may be a liquid or a paste.such as water or other solvents,with dessolved salts,acids or alkalis to impart ionic conductivity the electrolyte must have good ionic conductivity.other characteristics are nonreactivity with the electrode meterials,sefty in handling,low cost.
Type of cells
Primary Cells
the cell which we cannot recharge,once we use it,we have to replace it.many primary cells which the electrolyte there is no free or liquid electrolyte termed as dry cells.this type of primary battery is a convenient,lightweight usually inexpensive for electrical and electronic devices.
the advantages of primary batteries are good self life,high energy density at low to moderate discharge rate,little,low maintenance and ease of use.the example of primary cells are zinc-carbon,dry cell,zinc chloride cell,alkaline cells,mercury cell etc.
Secondary Cells
we can recharge this type of cells and batteries after discharge.in charging process,the electrical energy is injected to the cell by passing current in the opposite direction through it.so in this cells the electrical energy stored in the form of chemical energy.so therefore this type of cell is also called storage cells.
secondry batteries are following characterised.
- it has very high power density.
- it has high discharge rate
- flate discharge curve
- good low-temperature performance
the secondary cells are Lead-acid battery,Nickel-Cadmium battery,alkaline battery etc.
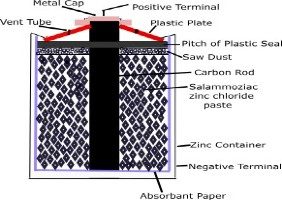
Dry Zinc-Carbon Cell
it is the top best primary cell.this cell also called lechlanche cell.the anode in this cell is mainly made of carbon rod,which is located in the centrally.cathode in this cell is mainly made of zinc cup lined with paper.
sal ammoniac zinc chloride past,manganese dioxide and carbon dust is filled in between carbon and zinc cup lined with paper.
reaction-when zinc atoms react with the electrolyte,the electrons are removed from the carbon rod.these electron accumulate on the zinc electrode.so therefore carbon rode lost electrons act as positive terminal.
ammonium chloride paste reacts with zinc to release hydrogen.
Zn+2NH_{4}CI\rightarrow Zncl_{2}+2NH_{3}+H_{2}
this hydrogen reacts with manganese dioxide as.
H_{2}+2MnO_{2}\rightarrow Mn_{2}+H_{2}O
Properties of Carbon-Zinc Cell
- the cell voltage for this type of cell is about 1.5 volts
- the internal resistance is about 0.1 to 0.4\Omega
- this cell is least expensive.
- this cell is portable and convenient to use
- the capacity 32Ah when discharge through 20\Omega resistance
- it is better to use this type of cell intermittently because in working condition,voltage drops due to polarisation.
Disadvantages of this Cell
- when cell not in use for long time,zinc gets attacked by the paste slowly and cell becomes useless.
- in this type of cell,hydrogen is released rapidly while the reaction of Mno_{2} is slow.for this reason,hydrogen makes a thin layer around the carbon road.this is called polarisation.these polarisation cause battery emf to drop.
Application
these cell are used in various field like,torch lights,telephone and telegraph systems,clocks,toyes,electronic circuits,radio,speaker etc.
Type of Cells Connection
there are three type of cells connection used
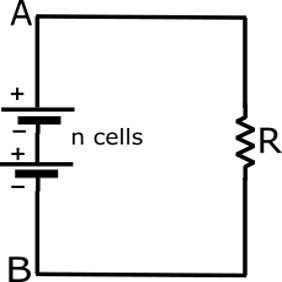
Series Connection
cells or batteries both must be connected in series to produced the required bettery voltage.when a number of cells are connected in such a way that the positive terminal of one cell is connected to the negative terminal of another cell and so on.this type of connecting cells is called series connection.this type of cells the load current does not exceed the discharge current of the cells.
Total emf of the battery
E=E_{1}+E_{2}+E_{3}+……E_{n}
and total internal resistance of the battery is equal to the sum of the internal resistance of the indivisual cells.the series cells connection gives strong current when the internal resistance is very low in comparison to the external resistance or load.
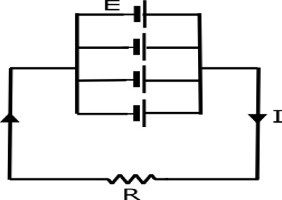
Parallel Connection
when a number of cells or batteries are connected in such a way that the positive terminals of all the cells or batteries are connected together and negative terminals of all the cells or batteries are connected togethers.this type of connecting cells or batteries are called parallel connection of cells and battery.in parallel connection the voltage is always equal to the emf of a cell
from ohm’s law
E=IR
I=\dfrac{E}{R}
if m cells each of emf E volts and internal resistance r ohms are connected in parallel with external circuit of resistance R ohm’s
then total internal resistance =\dfrac{1}{\dfrac{1}{r}+\dfrac{1}{r}+\ldots \ldots .m}
= \dfrac{1}{\dfrac{m}{r}}
= \dfrac{r}{m}
total resistance of the circuit = R+\dfrac{r}{m}
=\dfrac{mR+r}{m}
circuit current I=\dfrac{E}{\dfrac{mR+r}{m}}
=\dfrac{mE}{mR+r}
parallel cells or batteries connection gives strong current when the external resistance is very small in comparison to the internal resistance of the cells.
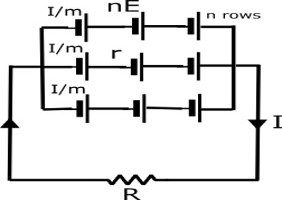
Grouped Cells Connection
when some cells or batteries are connected in series and many series connections are connected parallel,then this type of connection is called grouping of cells or batteries.
I am an engineer in a government department and also a blogger. I write posts on topics related to electrical and electronics engineering.
